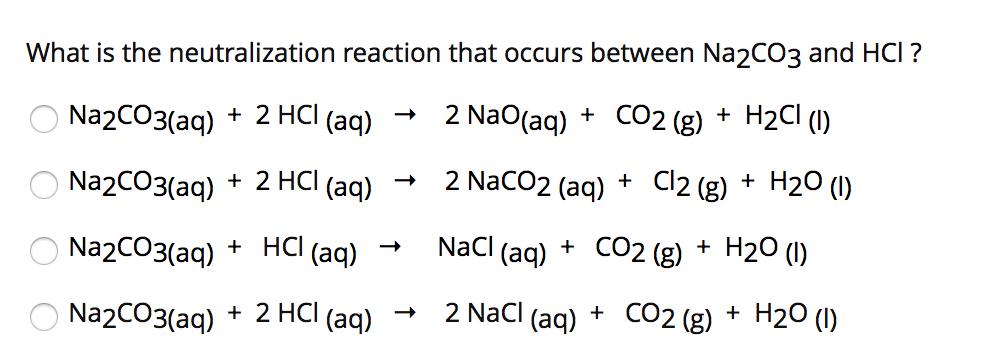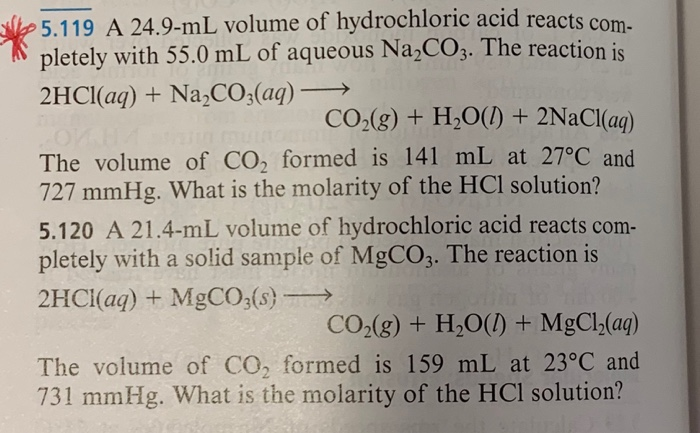in the titration of Na2CO3 by HCl using methyl orange indicator, thevolume required at the equivalence point will be if that of the acidrequired using phenolphthalein indicator is 10.0 ml:

Solved! How many liters of 0.53 M HCl is required to neutralize 0.78 g of sodium carbonate (Na2CO3)? (MM of Na2CO3 = 105. 99 g/mol) 𝟐𝑯𝑪𝒍 + 𝑵𝒂𝟐𝑪𝑶𝟑 → 𝟐𝑵𝒂𝑪𝒍 +

Net Ionic Equation for Na2CO3 + HCl | Sodium Carbonate + Hydrochloric Acid | Net Ionic Equation for Na2CO3 + HCl | Sodium Carbonate + Hydrochloric Acid Hello, Chemistry Enthusiasts! For today's
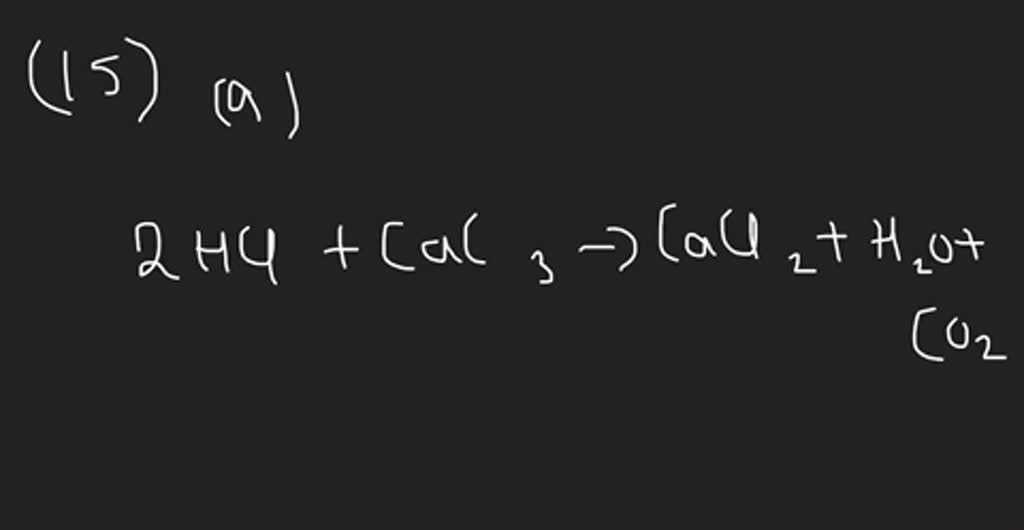
SOLVED: 2HCl+NaCO3 - - - - - > 2NaCl+H2CO3 ¿Cuántos gramos de H2CO3 se producen si reaccionan completamente 180 litros de HCl?

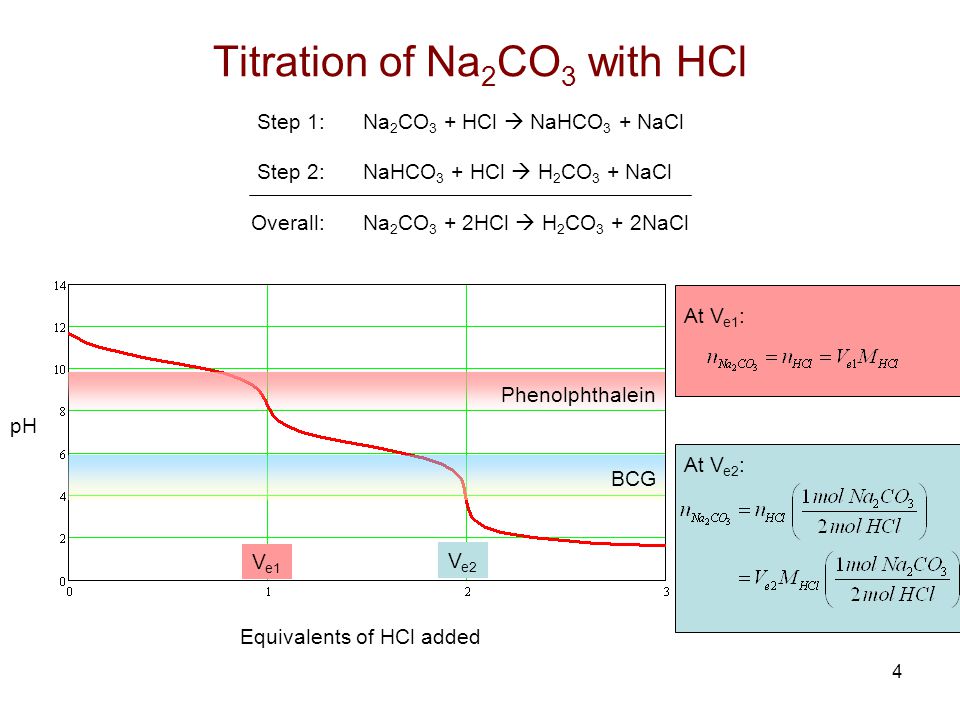
In the mixture of (NaHCO3 + Na2CO3) volume of HCl required is x mL with phenolphthalein indicator and y mL with methyl orange indicator in the same titration. Hence, volume for complete

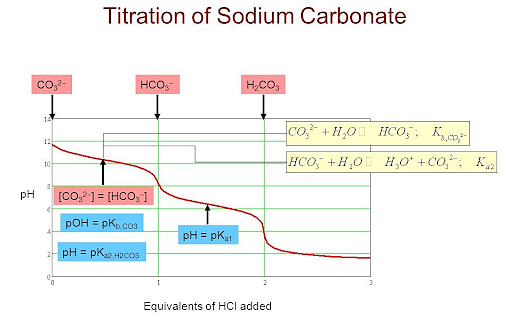
During the titration of sodium carbonate with H Cl, the dissolved carbonate ion will exist in three different forms; CO_3^{-2}, H CO_3^{-1}, and H_2 CO_3. During which part of the titration (initial,
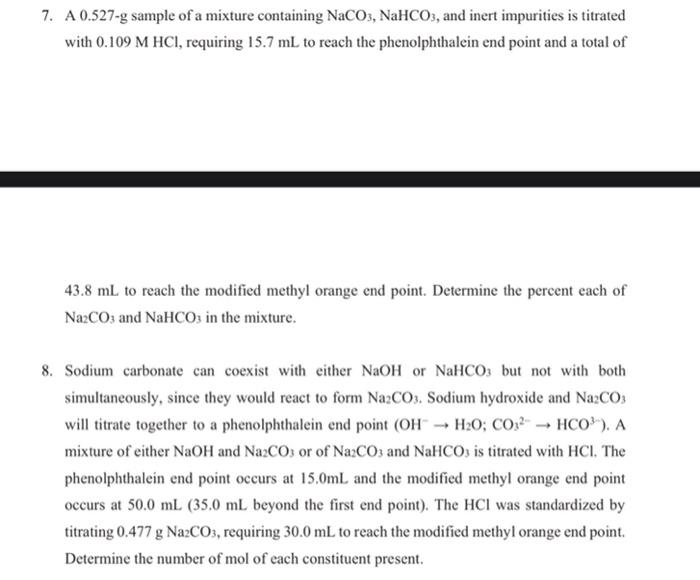
0.1 g of a solution containing Na2CO3 and NaHCO3 requires 10 ml, of 0.01 N HCl.neutralization using phenophthalein .wt