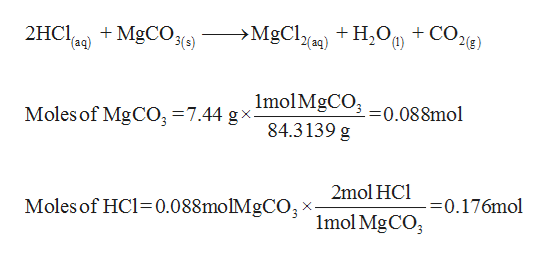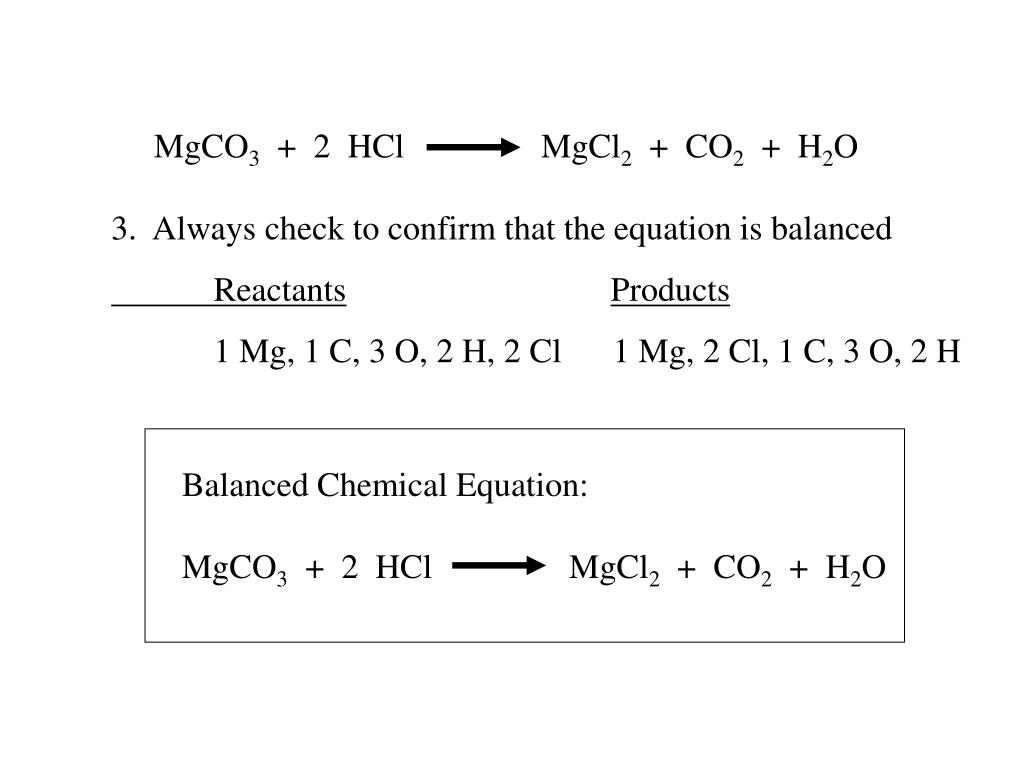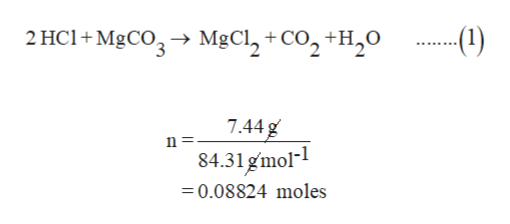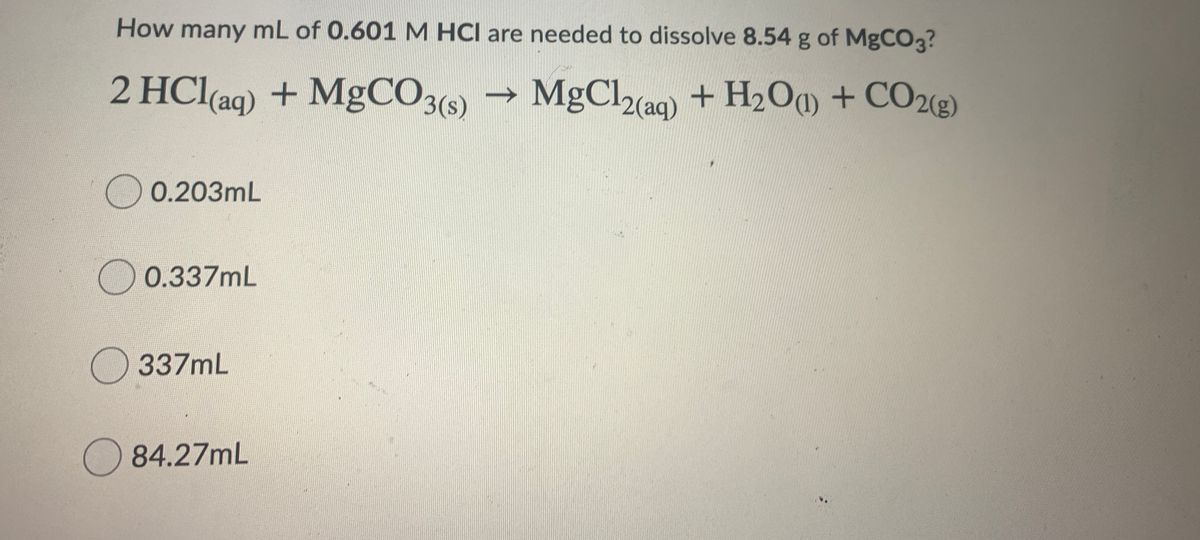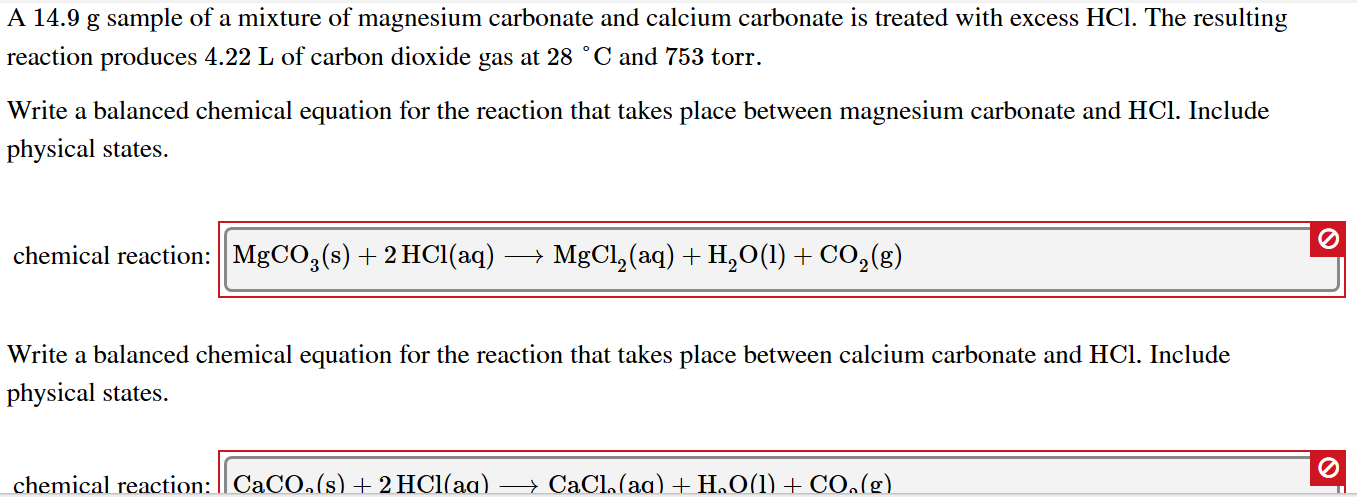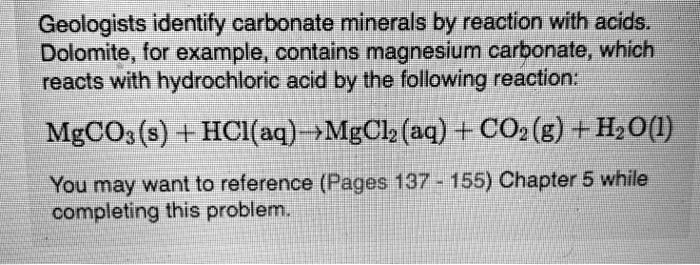
SOLVED: Geologists identify carbonate minerals by reaction with acids. Dolomite, for example, contains magnesium carbonate, which reacts with hydrochloric acid by the following reaction: MgCO3 (s) + HCl (aq) â†' MgCl2 (aq) +

How are we supposed to know that there's 2 compounds of HCl reacting? In other words, why is the product H2CO3 instead of HCO3- (because I'm reacting 1 HCl with 1 MgCO3) :
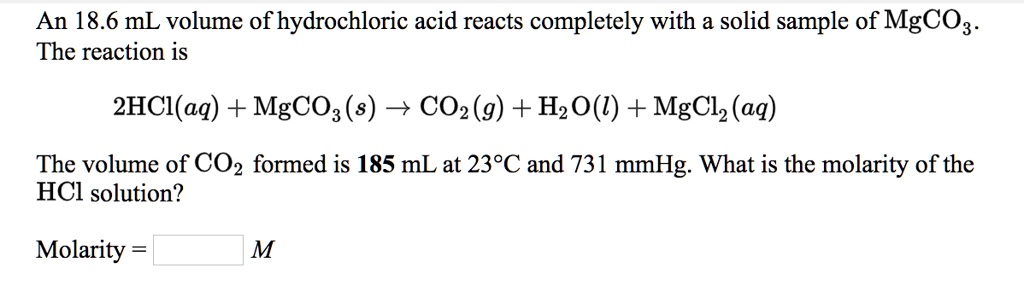
SOLVED: An 18.6 mL volume of hydrochloric acid reacts completely with a solid sample of MgCO3. The reaction is: 2HCl(aq) + MgCO3(s) = CO2(g) + H2O(l) + MgCl2(aq) The volume of CO2
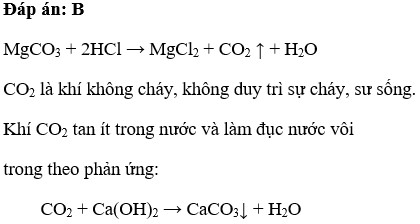
MgCO3 tác dụng với dung dịch HCl sinh ra A. Chất khí cháy được trong không khí B. Chất khí làm vẫn đục nước vôi trong

How are we supposed to know that there's 2 compounds of HCl reacting? In other words, why is the product H2CO3 instead of HCO3- (because I'm reacting 1 HCl with 1 MgCO3) :