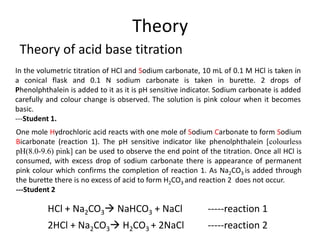
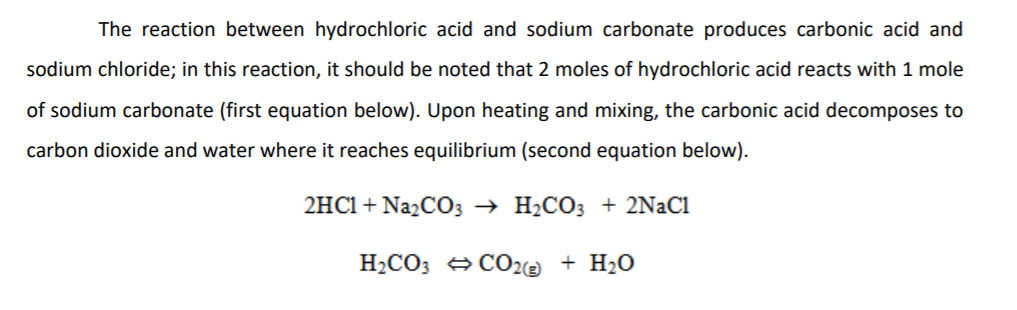
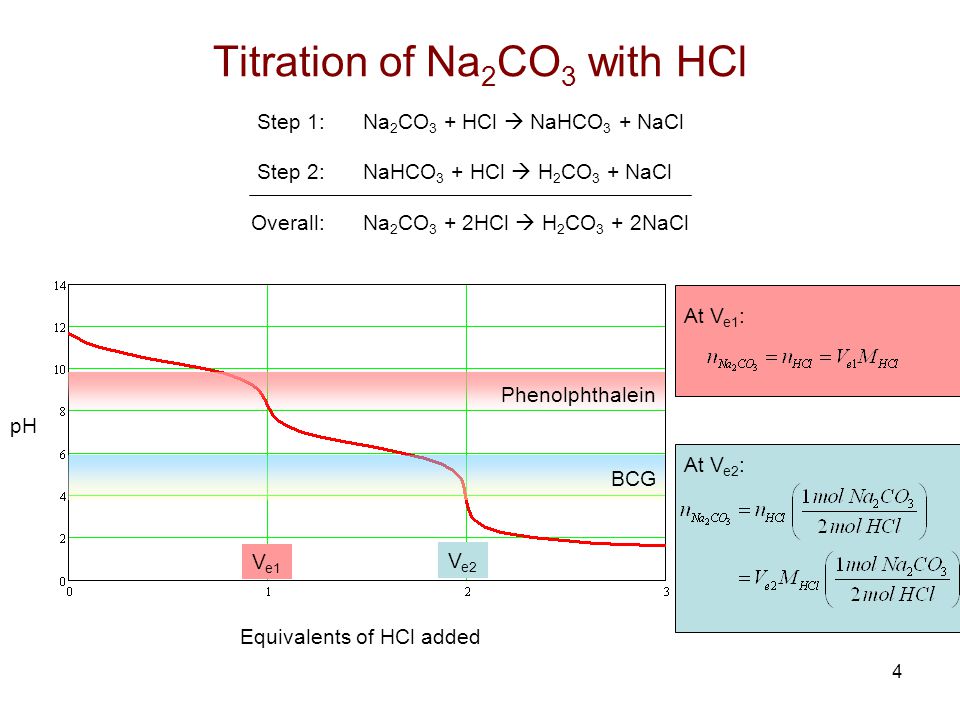
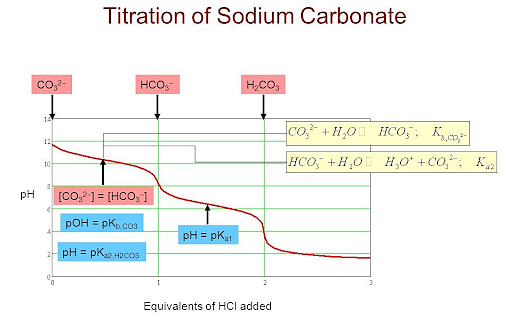
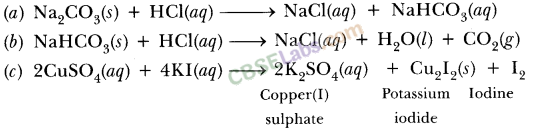in the titration of Na2CO3 by HCl using methyl orange indicator, thevolume required at the equivalence point will be if that of the acidrequired using phenolphthalein indicator is 10.0 ml:

The student will: be able to explain the experimental technique of titration. math calculate the molarity or volume of an unknown solution using the titration. - ppt download

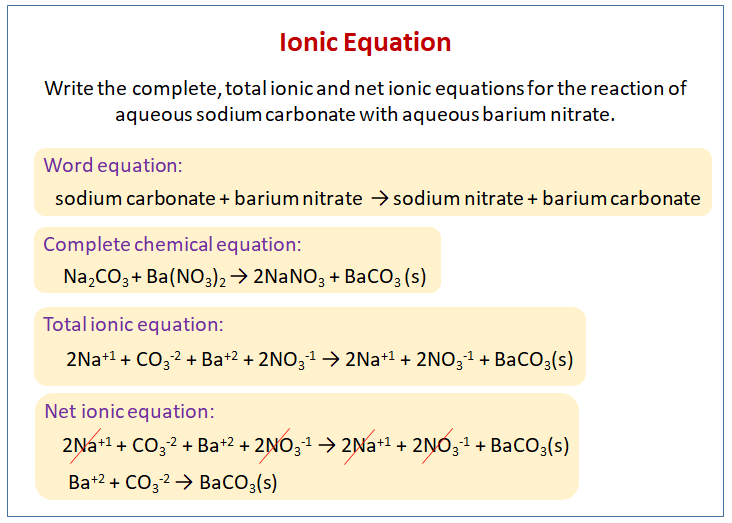
Sodium Carbonate + Hydrochloric Acid - Na2CO3 + HCl - Molecular Equations & Net Ionic Equations - YouTube

Question Video: Identifying the Observations of the Reaction between Hydrochloric Acid and Sodium Carbonate | Nagwa

SOLVED: 2. Na2CO3(aq) + HCl(aq) → NaCl(aq) + CO2(g) + H2O(l) Balanced Equation: Complete Ionic Equation: Net Ionic Equation:
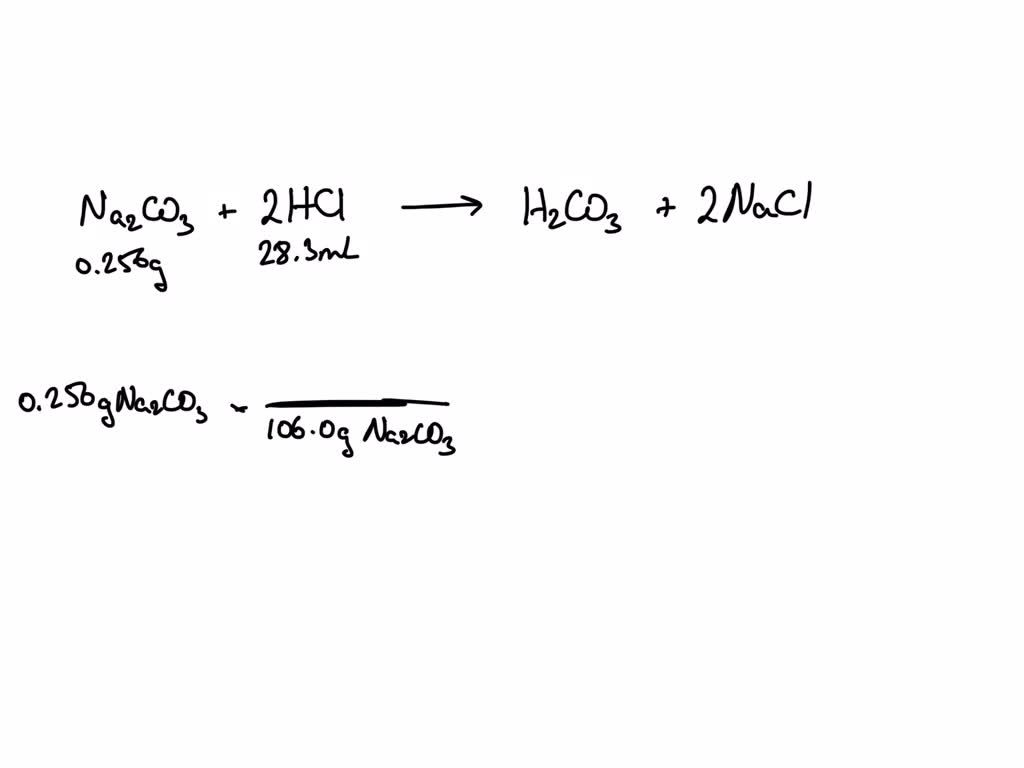
SOLVED: What is the molarity of a HCl solution if 28.3 mL of the solution are required to react with 0.256 g of the Na2CO3?

We can measure the concentration of HCl solution by its reaction with pure sodium carbonate. 2 H+ + Na2CO3 ? 2 Na+ + H2O + CO2 Complete reaction with 0.9639 0.0005 g of Na2CO3 required 28.20 0. | Homework.Study.com

In the mixture of (NaHCO3 + Na2CO3) volume of HCl required is x mL with phenolphthalein indicator and y mL with methyl orange indicator in the same titration. Hence, volume for complete
What volume of 0.25M Hcl is required to react completely with 22.6g of Na2co3 according to the equation Na2Co3+2Hcl=2Nacl=H20 (2) The molecular mass of organic compound is 78
What are reasons for getting two different concentration values of Na2CO3 when it was titrated with HCL using Phenolphthalein and Methyl Orange? - Quora