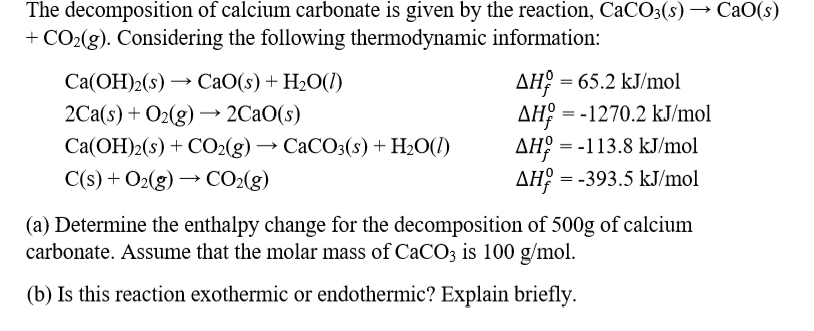
In the equilibrium CaCO3(s)Cao (s)+CO2(g) at 1073 K, the pressure of CO2 isfound to be 2.5 x 10 Pa. The equilibriumconstant for the reaction at 1073 K will be.(a) 0.25(c)25(b) 2.5(d) 250
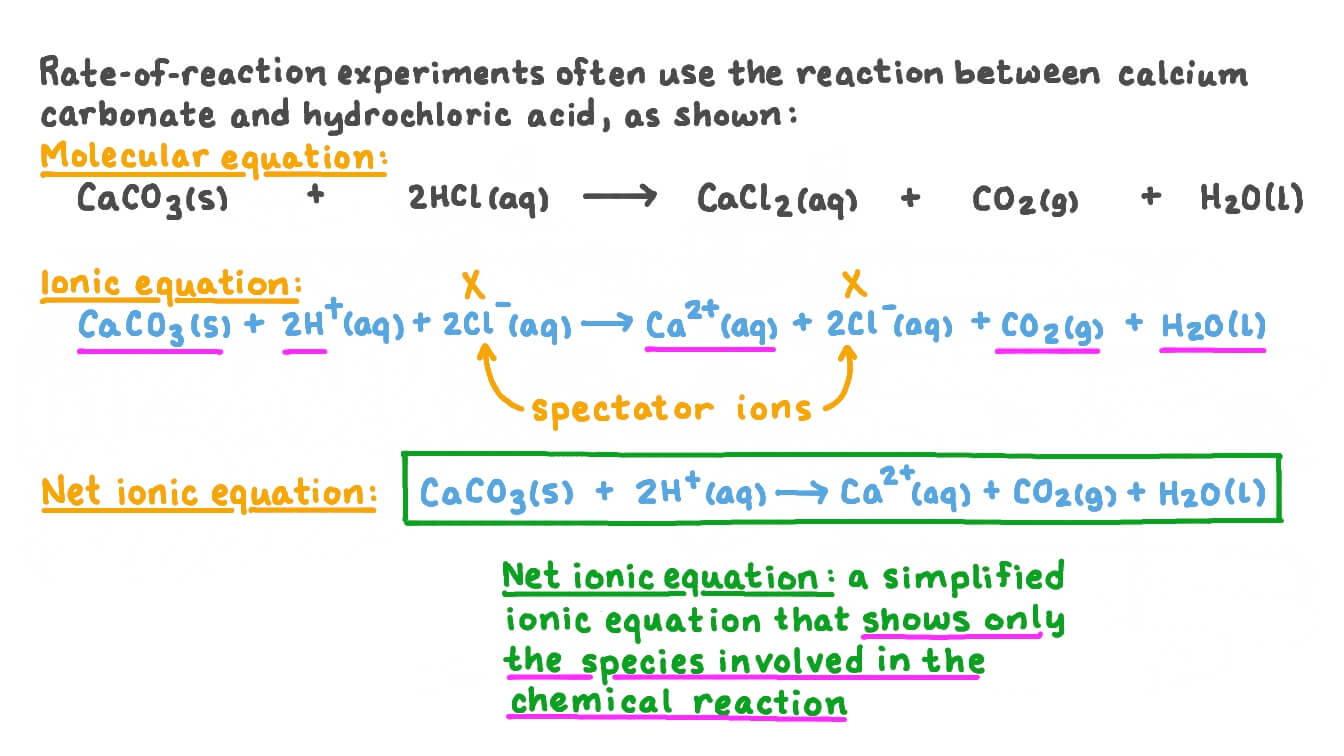
Question Video: Writing a Net Ionic Equation for the Reaction of Solid Calcium Carbonate with a Hydrochloric Acid Solution | Nagwa

How to Balance CaCO3 + HCl = CaCl2 + CO2 + H2O | How to Balance CaCO3 + HCl = CaCl2 + CO2 + H2O Hey there! Are you struggling with balancing

Reaction steps involved in the formation of the CaCO3 sol from calcium... | Download Scientific Diagram
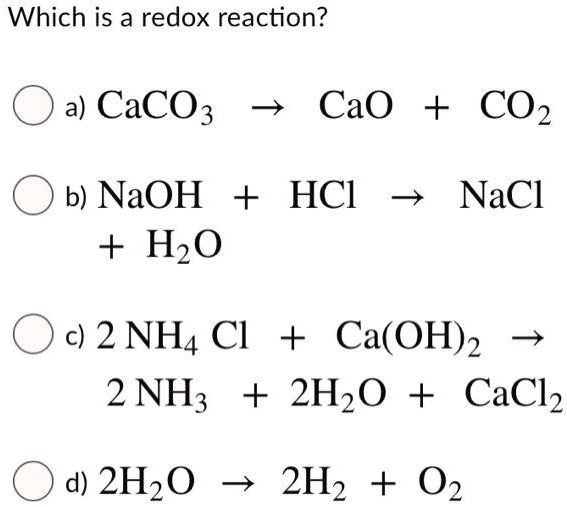
SOLVED: Which is a redox reaction? a) CaCO3 â†' CaO + CO2 b) NaOH + H2O â†' HCl + NaCl c) 2 NH4Cl + Ca(OH)2 â†' 2 NH3 + 2H2O + CaCl2 d) 2H2O â†' 2H2 + O2

How to Balance Ca(HCO3)2 = CaCO3 + CO2 + H2O (Decomposition of Calcium hydrogen carbonate) - YouTube
If 220 grams of calcium oxide (CaO) reacts with 50L of carbon dioxide (CO2), what mass of calcium carbonate (CaCO3) is produced? - Quora
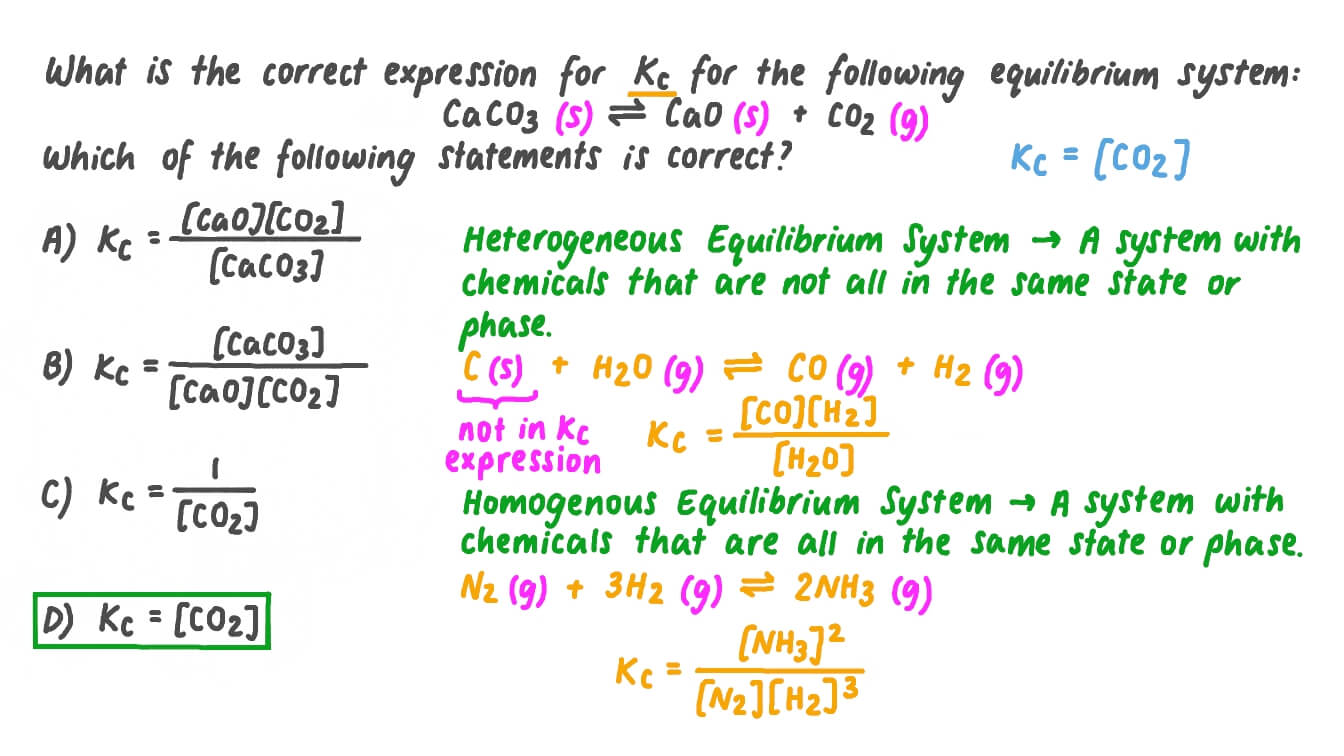
Question Video: Determining the Expression for the Equilibrium Constant for the Decomposition of Calcium Carbonate | Nagwa
CO2 mineralization into different polymorphs of CaCO3 using an aqueous-CO2 system - RSC Advances (RSC Publishing)
![SOLVED: What is the expression for the equilibrium constant? CaO(s) + CO2(g) ⇌ CaCO3(s) [CaO][CO2] / [CaCO3] b) Keq = [CaCO3] / [CaO][CO2] c) Keq = [ CO2] [CO2] Keq = [CO2] Keq = [ SOLVED: What is the expression for the equilibrium constant? CaO(s) + CO2(g) ⇌ CaCO3(s) [CaO][CO2] / [CaCO3] b) Keq = [CaCO3] / [CaO][CO2] c) Keq = [ CO2] [CO2] Keq = [CO2] Keq = [](https://cdn.numerade.com/ask_images/928a15231b094c709901e0e2efd39d00.jpg)
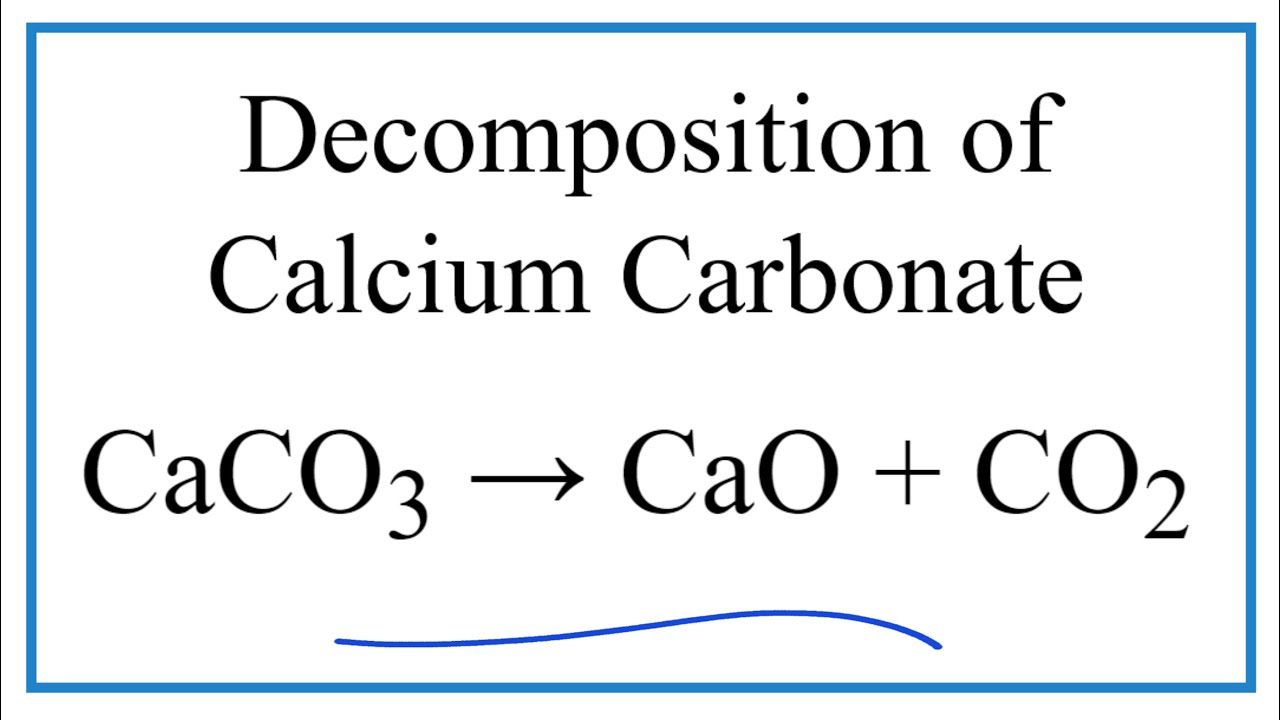

![ANSWERED] Given the following chemical equation CaO... - Physical Chemistry ANSWERED] Given the following chemical equation CaO... - Physical Chemistry](https://media.kunduz.com/media/sug-question-candidate/20220517131515311837-4392391.jpg)