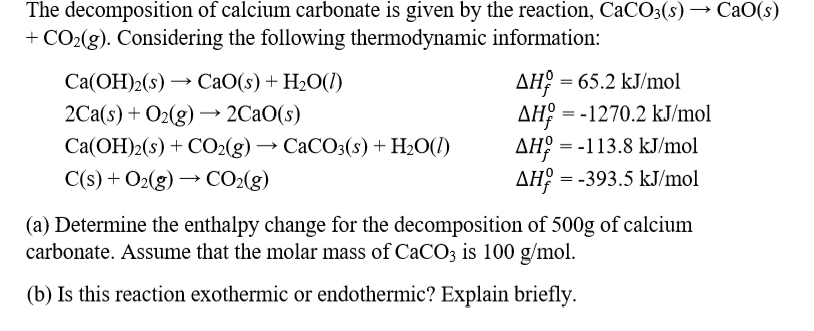
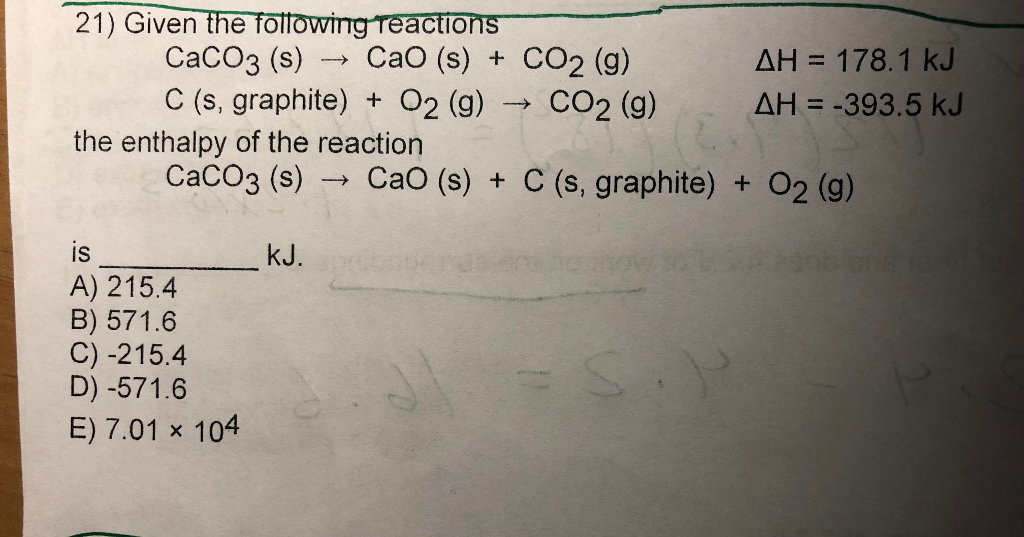CaCO3(s) CaO(s) + CO₂(g) 4 3.25 mol CaCO3 decomposes according to the reaction above. What volume of CO2 - Brainly.com
Foods | Free Full-Text | Stability, Microstructure, and Rheological Properties of CaCO3 S/O/W Calcium-Lipid Emulsions

Real-Time Observation of CaCO3 Mineralization in Highly Supersaturated Graphene Liquid Cells | ACS Omega
The partial pressure of carbon dioxide in the reaction CaCO3(s) ⇌ CaO(s) + CO2(g) is 1.017 x 10^-3 atm at 500° C. - Sarthaks eConnect | Largest Online Education Community

The industrial production of lime ( CaO ) from calcium carbonate is accomplished via the following - Brainly.com

SOLVED: Consider the following reaction: CaCO3(s)→CaO(s)+CO2(g). Estimate ΔG∘ for this reaction at each of the following temperatures. (Assume that ΔH∘ and ΔS∘ do not change too much within the given temperature range.)
CaO(s) + CO2(g) → CaCO3(s) + heat What is the total mass of CO2(s) needed to produce 300. grams of CaCO3(s)? - Quora
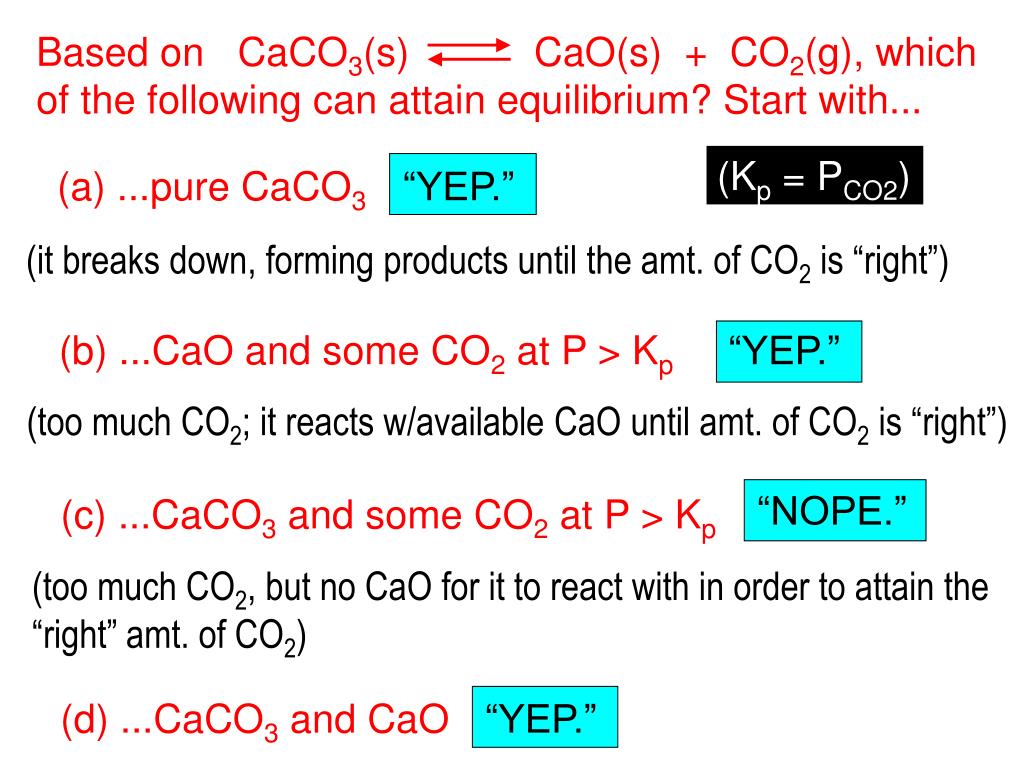
CaCO3(s) → ← CaO(s) + CO2(g) When heated, calcium carbonate decomposes according to the equation above. In a study of the


![ANSWERED] Given the following chemical equation CaO... - Physical Chemistry ANSWERED] Given the following chemical equation CaO... - Physical Chemistry](https://media.kunduz.com/media/sug-question-candidate/20220517131515311837-4392391.jpg)