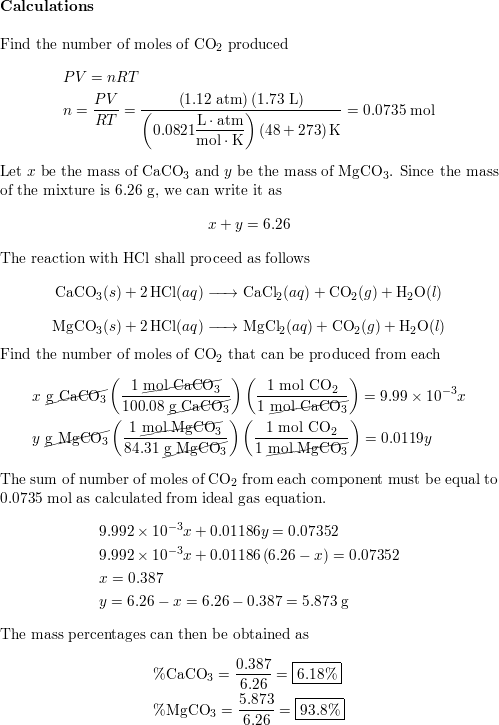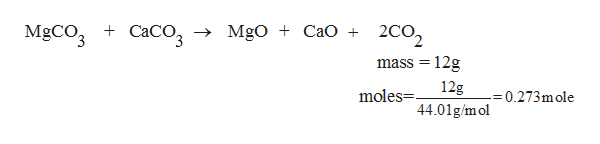20g of caco3 and mgco3 mixture was heated produced 112l of co2 calculate age composition of mixture eoohtxmm -Chemistry - TopperLearning.com
1.84 gm mixture of CaCO3; & MgCO3; was heated to a constant weight. The constant weight of residue was found to be 0.96 gm. Calculate composition of initial mixture? - CHEMISTRY FOR NEET - Quora
24 1 gm of a mixture of CaCO3 and MgCO3 is reacted with access dil. HCl. Thus produced CO2 whose volume is 240 ml in S.T.P. Calculate the conjugated percentage of the mixture.

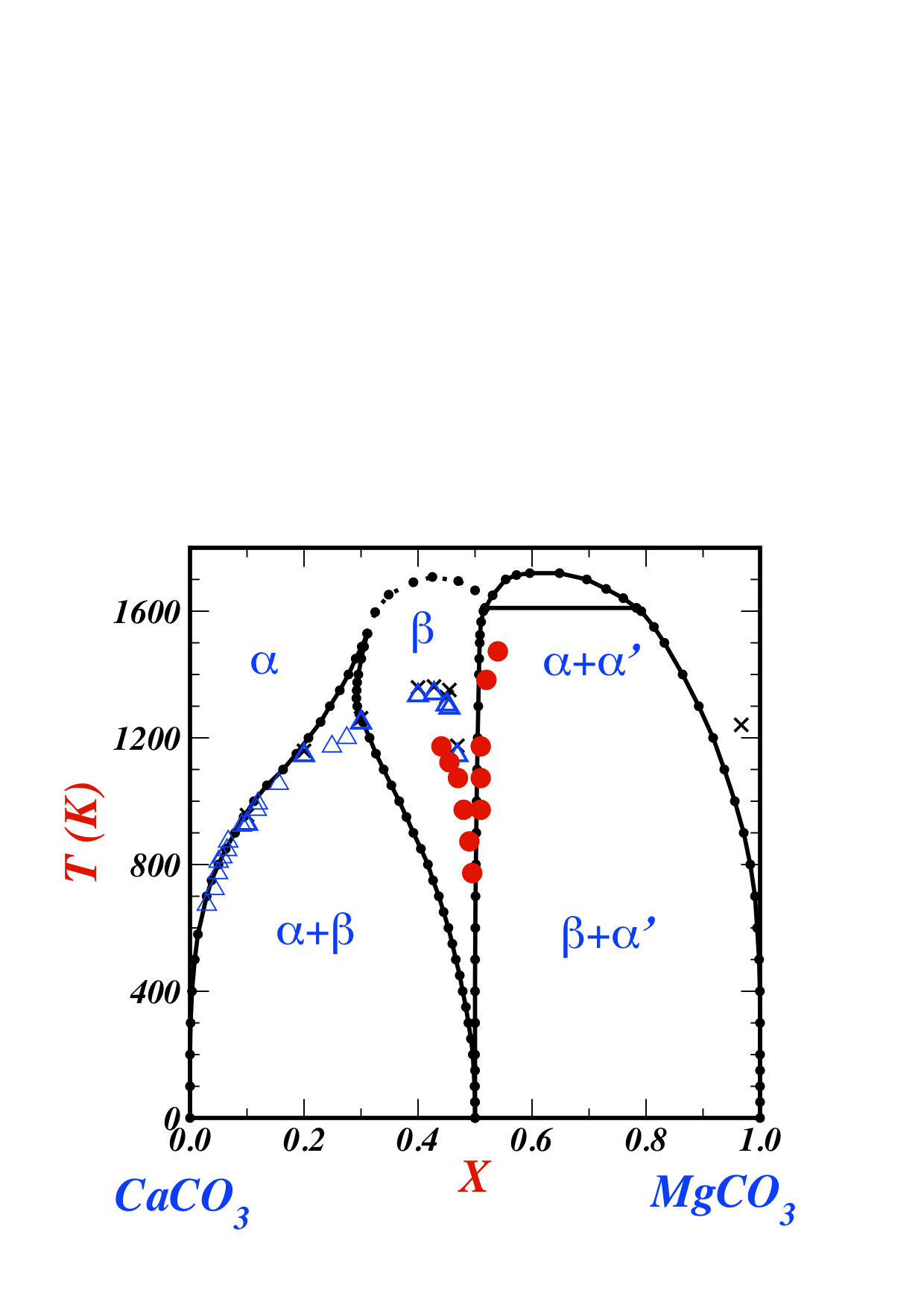
Minerals | Free Full-Text | The K2CO3–CaCO3–MgCO3 System at 6 GPa: Implications for Diamond Forming Carbonatitic Melts

CaCO3-MgCO3-SrCO3 molar ratio plot illustrating the compositions of the... | Download Scientific Diagram
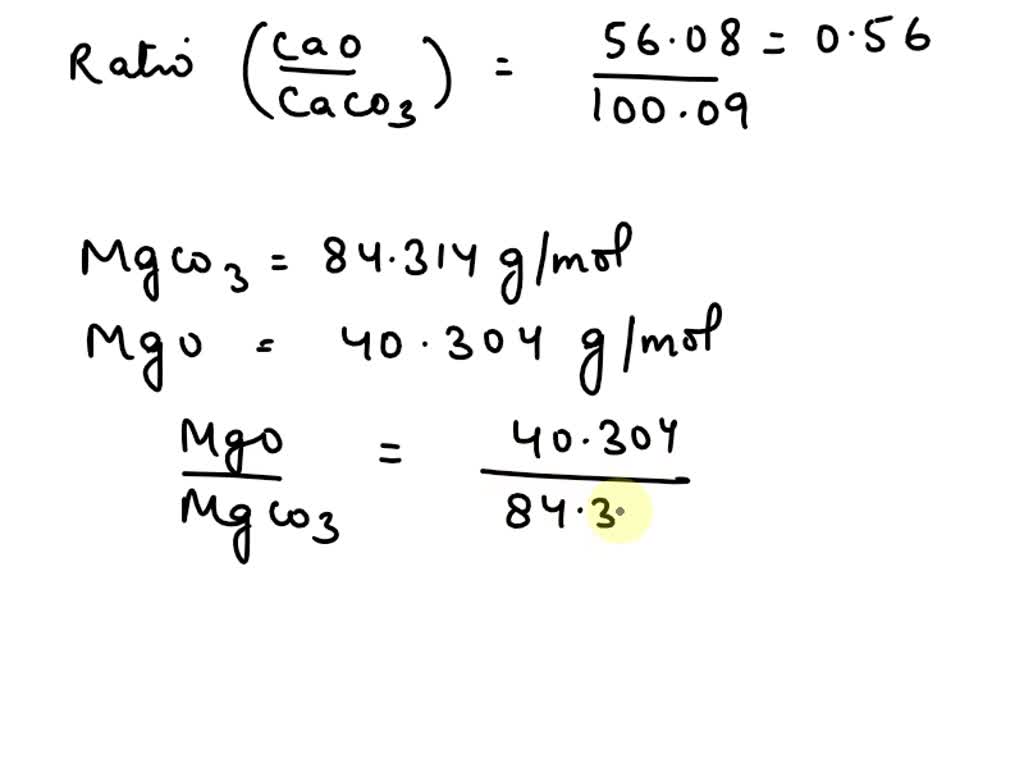
SOLVED: A sample containing only CaCO3 (100.09 g/mol) and MgCO3 (84.314 g/mol) is ignited to CaO (56.08 g/mol) and MgO (40.304 g/mol). The mixture of oxides weighs exactly half as much as

18.4 g of a mixture of calcium carbonate and magnesium carbonate, on heating, gives 4.0 g of magnesium oxide. The volume of CO2 produced at STP in this process is:
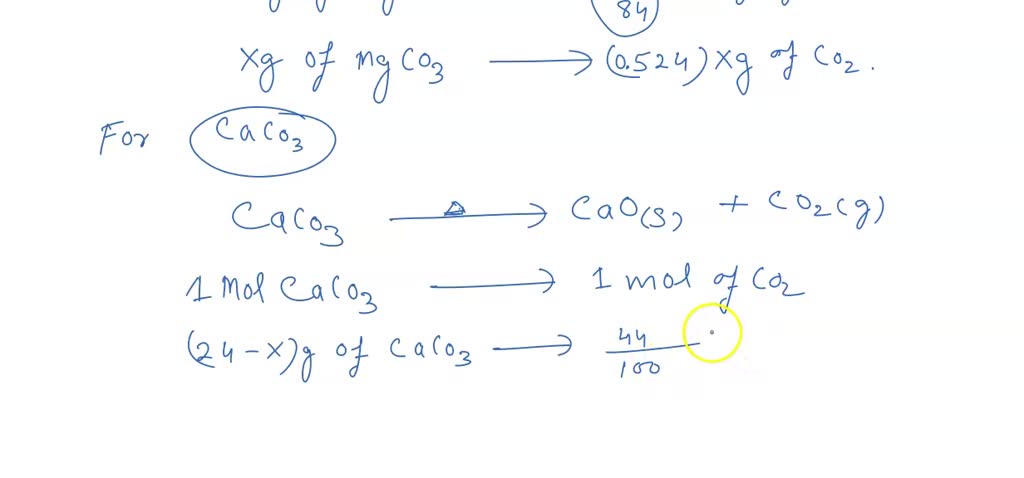
SOLVED: When a solid mixture of MgCO3 and CaCO3 is heated strongly, carbon dioxide gas is given off and a solid mixture of MgO and CaO is obtained. If a 24.00 g

Minerals | Free Full-Text | The K2CO3–CaCO3–MgCO3 System at 6 GPa: Implications for Diamond Forming Carbonatitic Melts

a Shows a quaternary diagram of the MgCO3–CaCO3–SrCO3–BaCO3 system with... | Download Scientific Diagram

A sample containing only caco3 and mgco3 is ignited to cao and mgo. The mixture of oxides produced at - Brainly.in
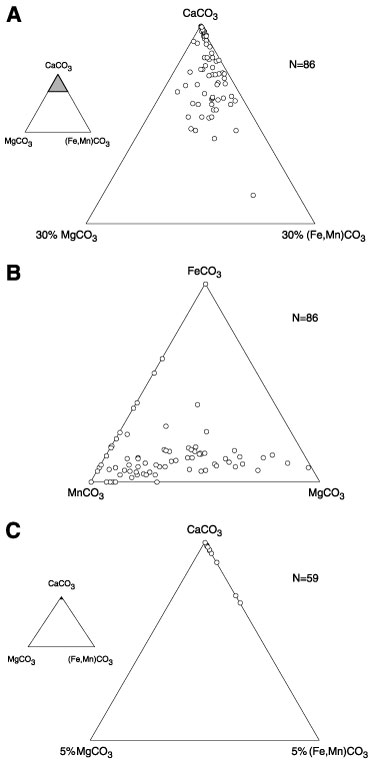
Figure 2. Electron microprobe analyses of carbonate minerals. A. Relative contributions (wt%) of CaCO3, MgCO3, and (Fe + Mn)CO3 to the overall mineral analysis for calcite. Eighty-six analyses are plotted. The inset