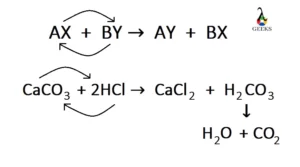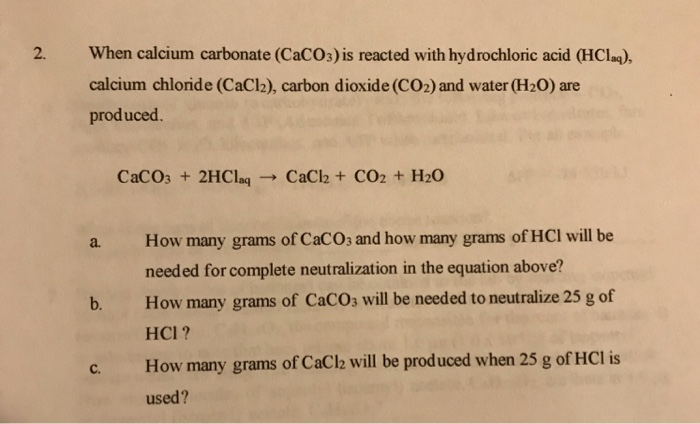What mass of calcium carbonate can 2000. mL of a 1.50M aqueous HCl solution dissolve? 2 HCl(aq) + CaCO3(s) à CaCl2(aq) + CO2(g) + H2O(l) - Quora
40. Consider the reaction CaCO3+2HCL (l) 》CaCl2+CO2+H2O (l).what mass of CaCO3 is required to react with 20mL 1M HCL?

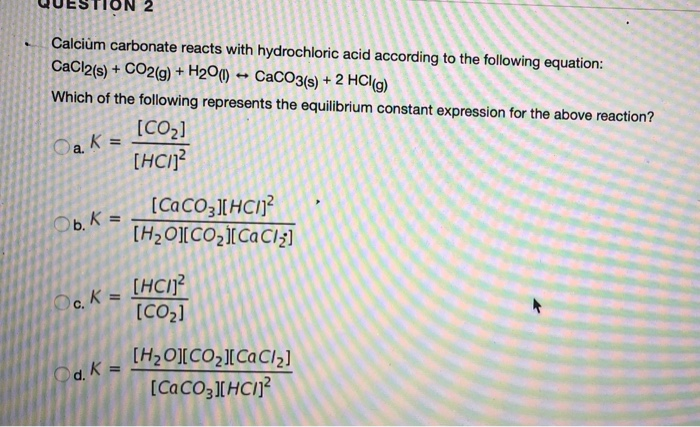
Which of the following reactions is balanced? A.CaCl2 + 2H2CO3 = CaCO3 + HCI B.2C2Cl2 + H2CO3 +CaCO3 + - Brainly.com

Question Video: Calculating the Average Rate of Reaction of Hydrochloric Acid with Calcium Carbonate | Nagwa
16. In a chemical reaction, caco3+2hcl= cacl2 +co2+h2o. 25ml hcl and 0.75M Calculate the amount of caco3

Balance the following equations : (a) Caco, (s) + HCl (aq) + CaCl, (aq) + H2O (1) + CO, (g) (b) Zn (s) + HCl - Brainly.in
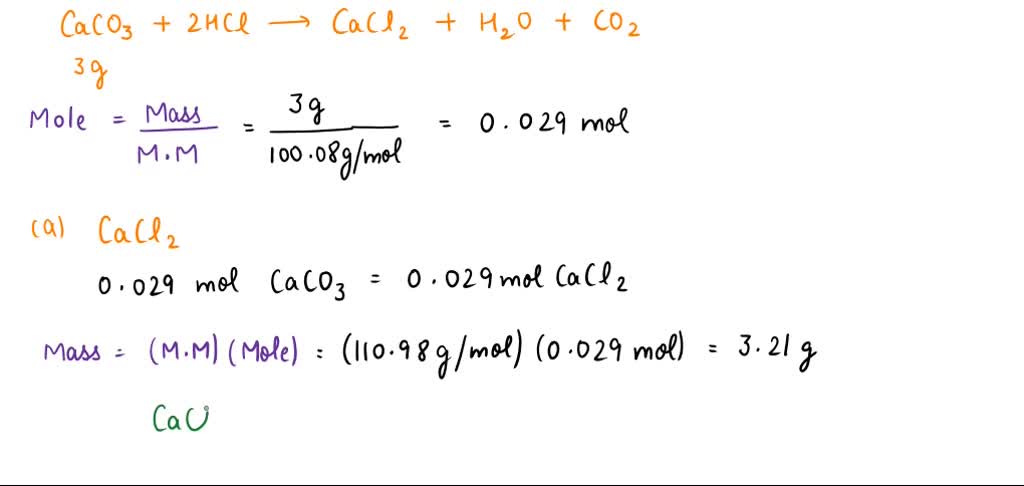
SOLVED: IF 3.00g of limestone reacted according to the equation CaCO3 + 2 HCl = CaCl 2 + H2O + CO2. What masses of the following would be produced? Calcium Chloride Carbon Dioxide Water

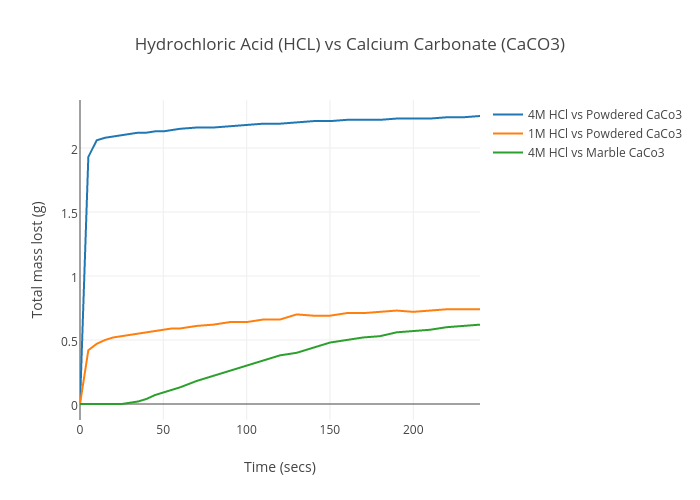
Amount of dissolved calcium carbonate in different chemical reagent systems | Download Scientific Diagram
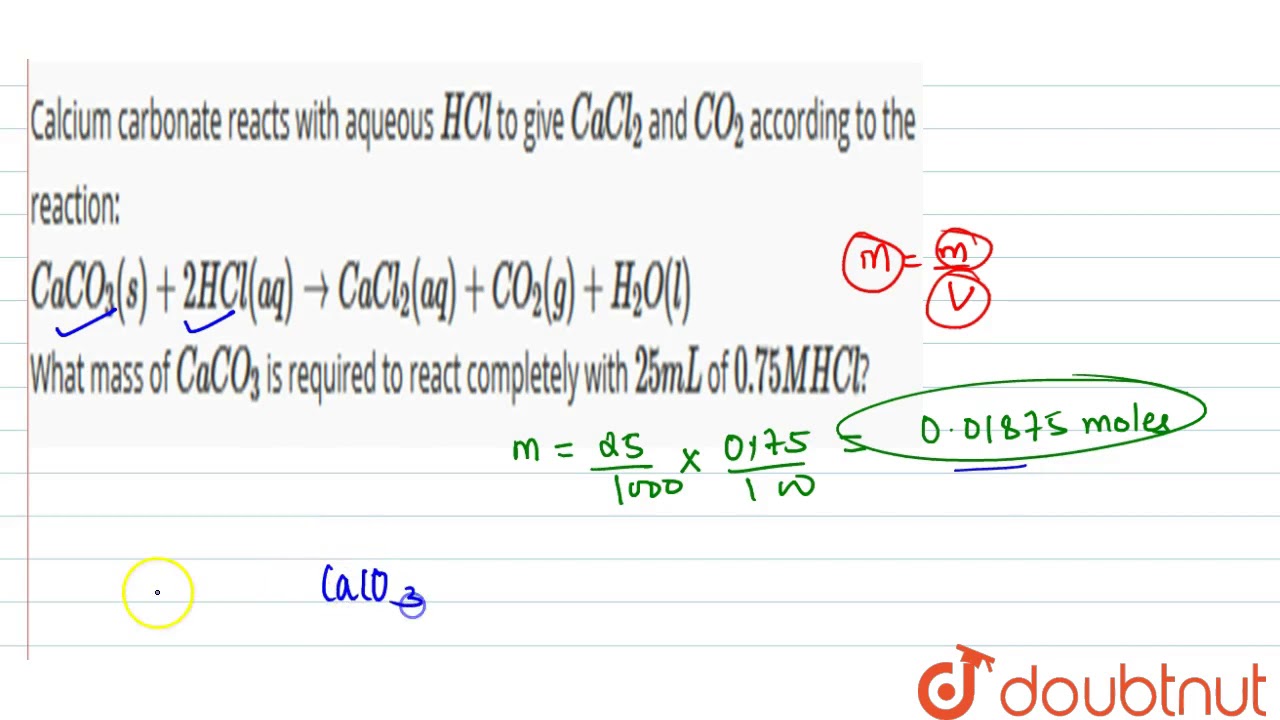
Calcium carbonate reacts with aqueous `HCl` to give `CaCl_(2)` and `CO_(2)` according to the rea... - YouTube

SOLVED: If 2.5g of CaCO3 is mixed with 2.0 g of HCl to complete the reaction CaCO3 + 2HCl ——–> CaCl2 +CO2 +H2O, What is limiting and what amount of CO2 will

Question Video: Writing a Net Ionic Equation for the Reaction of Solid Calcium Carbonate with a Hydrochloric Acid Solution | Nagwa