
The experimental enthalpies of solution of Y, BaCO3 and CoCl2·4.24H2O... | Download Scientific Diagram

SOLVED: Write the net ionic equation for the reaction that occurs when excess hydrochloric acid (aq) and barium carbonate are combined. Write the net ionic equation for the reaction that occurs when




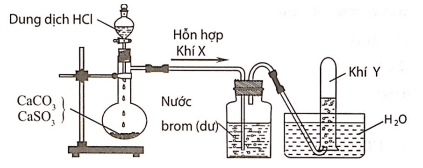






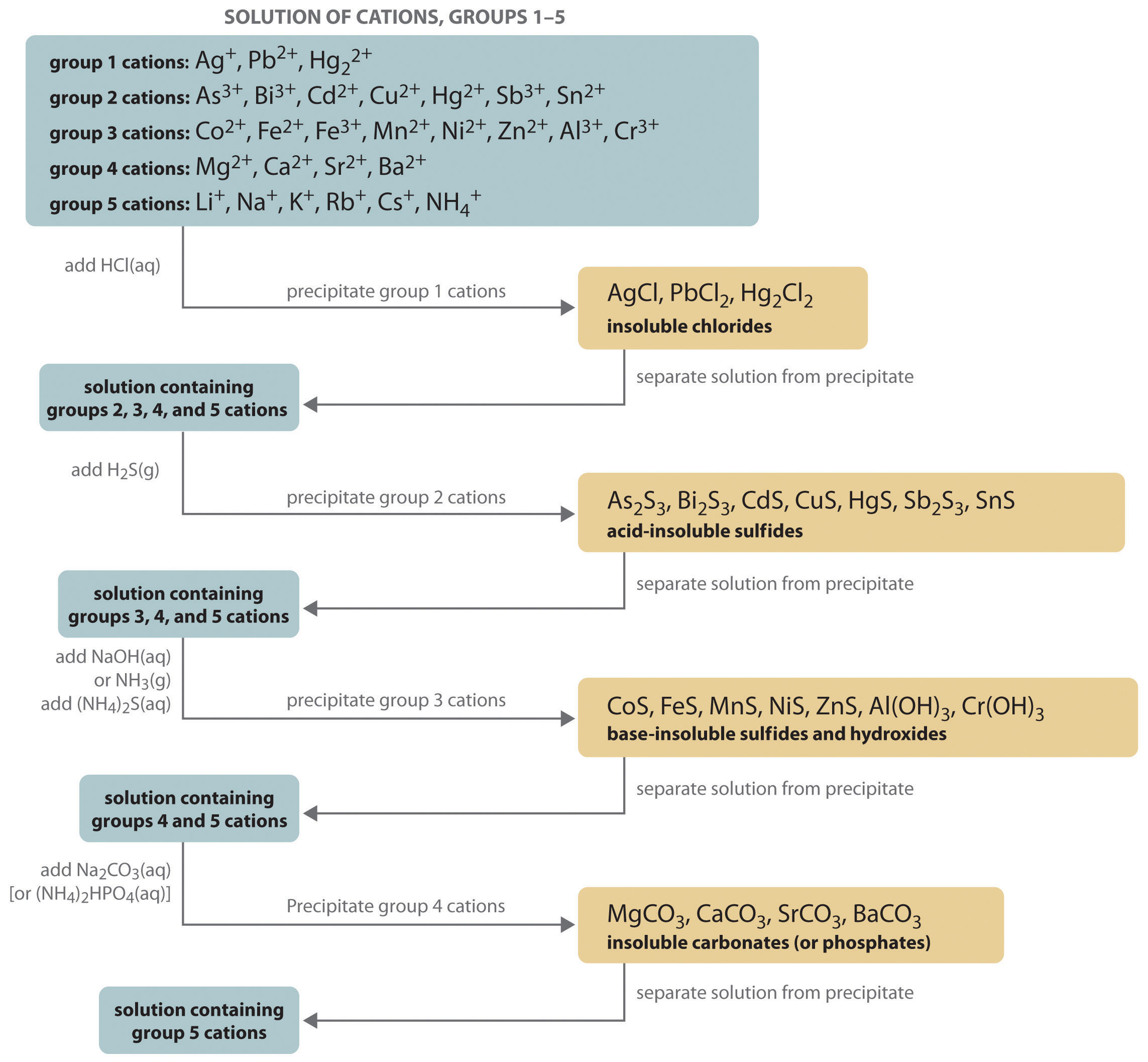


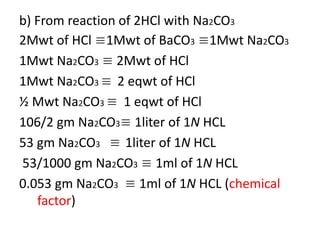
![LiAlH[Ot-Bu]3 For The Reduction of Acid Halides To Aldehydes – Master Organic Chemistry LiAlH[Ot-Bu]3 For The Reduction of Acid Halides To Aldehydes – Master Organic Chemistry](https://cdn.masterorganicchemistry.com/wp-content/uploads/2022/11/0-LiAlHOtBu3-selective-reagent-for-reduction-of-acid-halides-to-aldehydes.gif)




